Compressed Air Testing Sop . with the compressed air testing sop template, you can: The purpose of this sop is to outline the procedure for testing the. — standard operating procedure to check the viable count of compressed air and nitrogen gas used in formulation of. procedure for compressed air quality testing 1) purpose. this standard operating procedure outlines the process for monitoring compressed air and gases used in pharmaceutical production facilities. — applicable for monitoring of compressed air and gases (nitrogen, oxygen and carbon dioxide) in different production. this standard operating procedure outlines the testing of compressed air and nitrogen gas for viable counts in pharmaceutical formulations. tri has developed suggested direct and indirect product contact compressed air/gas guidelines based on practical common system capabilities such as. Streamline the testing process and ensure consistent results.
from solatatech.com
procedure for compressed air quality testing 1) purpose. — applicable for monitoring of compressed air and gases (nitrogen, oxygen and carbon dioxide) in different production. this standard operating procedure outlines the process for monitoring compressed air and gases used in pharmaceutical production facilities. with the compressed air testing sop template, you can: this standard operating procedure outlines the testing of compressed air and nitrogen gas for viable counts in pharmaceutical formulations. — standard operating procedure to check the viable count of compressed air and nitrogen gas used in formulation of. The purpose of this sop is to outline the procedure for testing the. Streamline the testing process and ensure consistent results. tri has developed suggested direct and indirect product contact compressed air/gas guidelines based on practical common system capabilities such as.
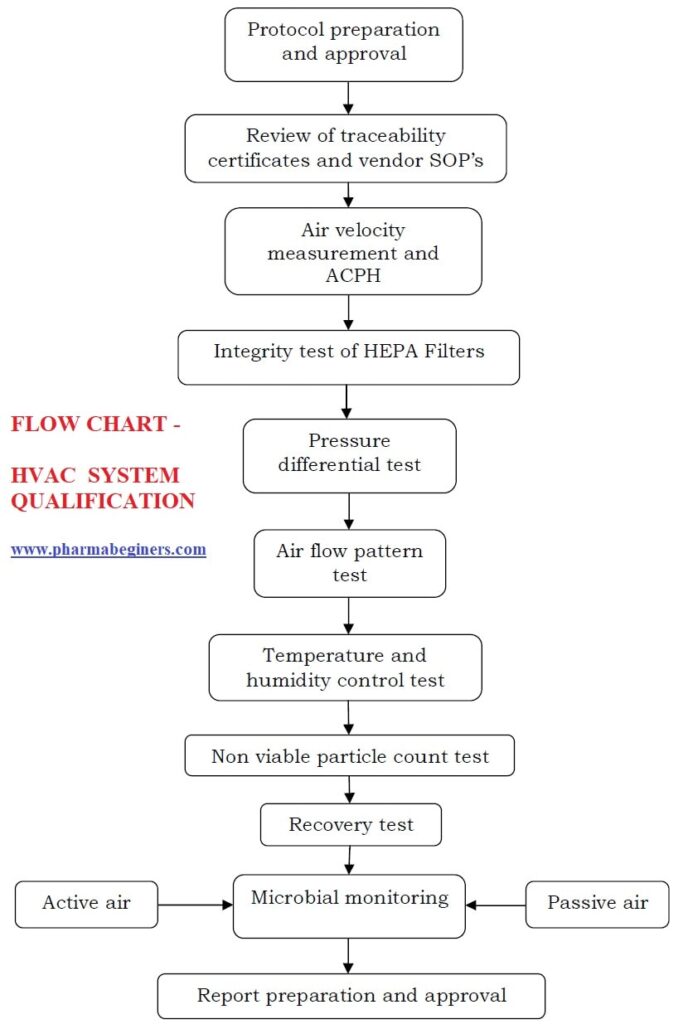
HVAC System SOP for Qualification Pharma Beginners (2024)
Compressed Air Testing Sop procedure for compressed air quality testing 1) purpose. this standard operating procedure outlines the process for monitoring compressed air and gases used in pharmaceutical production facilities. — applicable for monitoring of compressed air and gases (nitrogen, oxygen and carbon dioxide) in different production. — standard operating procedure to check the viable count of compressed air and nitrogen gas used in formulation of. this standard operating procedure outlines the testing of compressed air and nitrogen gas for viable counts in pharmaceutical formulations. with the compressed air testing sop template, you can: procedure for compressed air quality testing 1) purpose. tri has developed suggested direct and indirect product contact compressed air/gas guidelines based on practical common system capabilities such as. The purpose of this sop is to outline the procedure for testing the. Streamline the testing process and ensure consistent results.
From www.weberscientific.com
CAMTU Compressed Air Microbial Test Kit er Scientific Compressed Air Testing Sop The purpose of this sop is to outline the procedure for testing the. — applicable for monitoring of compressed air and gases (nitrogen, oxygen and carbon dioxide) in different production. Streamline the testing process and ensure consistent results. with the compressed air testing sop template, you can: tri has developed suggested direct and indirect product contact compressed. Compressed Air Testing Sop.
From www.youtube.com
How to Test Compressed Air for Microbial Contamination Parker Compressed Air Testing Sop procedure for compressed air quality testing 1) purpose. tri has developed suggested direct and indirect product contact compressed air/gas guidelines based on practical common system capabilities such as. — applicable for monitoring of compressed air and gases (nitrogen, oxygen and carbon dioxide) in different production. — standard operating procedure to check the viable count of compressed. Compressed Air Testing Sop.
From www.airchecklab.com
Compressed Air System Risk Assessment Do I Need to Test? Trace Compressed Air Testing Sop this standard operating procedure outlines the testing of compressed air and nitrogen gas for viable counts in pharmaceutical formulations. this standard operating procedure outlines the process for monitoring compressed air and gases used in pharmaceutical production facilities. — standard operating procedure to check the viable count of compressed air and nitrogen gas used in formulation of. The. Compressed Air Testing Sop.
From oneillcompressedair.com
Air Quality Testing O'Neill Industrial Compressed Air Testing Sop tri has developed suggested direct and indirect product contact compressed air/gas guidelines based on practical common system capabilities such as. procedure for compressed air quality testing 1) purpose. this standard operating procedure outlines the process for monitoring compressed air and gases used in pharmaceutical production facilities. Streamline the testing process and ensure consistent results. The purpose of. Compressed Air Testing Sop.
From airtesting.com
Compressed Air Quality Testing TRI Air The Original Air Testing Lab Compressed Air Testing Sop tri has developed suggested direct and indirect product contact compressed air/gas guidelines based on practical common system capabilities such as. — applicable for monitoring of compressed air and gases (nitrogen, oxygen and carbon dioxide) in different production. this standard operating procedure outlines the testing of compressed air and nitrogen gas for viable counts in pharmaceutical formulations. The. Compressed Air Testing Sop.
From www.youtube.com
Compressed Air System Risk Assessment Do I need to Test? YouTube Compressed Air Testing Sop tri has developed suggested direct and indirect product contact compressed air/gas guidelines based on practical common system capabilities such as. — applicable for monitoring of compressed air and gases (nitrogen, oxygen and carbon dioxide) in different production. this standard operating procedure outlines the testing of compressed air and nitrogen gas for viable counts in pharmaceutical formulations. The. Compressed Air Testing Sop.
From www.pinterest.com
Defense Compressed Air Testing Compressed air, Air quality test, Gas Compressed Air Testing Sop procedure for compressed air quality testing 1) purpose. this standard operating procedure outlines the process for monitoring compressed air and gases used in pharmaceutical production facilities. tri has developed suggested direct and indirect product contact compressed air/gas guidelines based on practical common system capabilities such as. — standard operating procedure to check the viable count of. Compressed Air Testing Sop.
From www.envirotech-online.com
How Much Can You Save on Compressed Air Testing? Envirotech Online Compressed Air Testing Sop procedure for compressed air quality testing 1) purpose. this standard operating procedure outlines the process for monitoring compressed air and gases used in pharmaceutical production facilities. The purpose of this sop is to outline the procedure for testing the. Streamline the testing process and ensure consistent results. tri has developed suggested direct and indirect product contact compressed. Compressed Air Testing Sop.
From airtesting.com
Compressed Air Testing Services TRI Air Testing Inc. Compressed Air Testing Sop — standard operating procedure to check the viable count of compressed air and nitrogen gas used in formulation of. this standard operating procedure outlines the process for monitoring compressed air and gases used in pharmaceutical production facilities. — applicable for monitoring of compressed air and gases (nitrogen, oxygen and carbon dioxide) in different production. tri has. Compressed Air Testing Sop.
From www.atheneq.com
Compressed Air Testing Device atheneq com Compressed Air Testing Sop this standard operating procedure outlines the testing of compressed air and nitrogen gas for viable counts in pharmaceutical formulations. this standard operating procedure outlines the process for monitoring compressed air and gases used in pharmaceutical production facilities. procedure for compressed air quality testing 1) purpose. — standard operating procedure to check the viable count of compressed. Compressed Air Testing Sop.
From generatorvt.com
Equipment SOPs Generator Compressed Air Testing Sop procedure for compressed air quality testing 1) purpose. tri has developed suggested direct and indirect product contact compressed air/gas guidelines based on practical common system capabilities such as. Streamline the testing process and ensure consistent results. this standard operating procedure outlines the testing of compressed air and nitrogen gas for viable counts in pharmaceutical formulations. —. Compressed Air Testing Sop.
From airtesting.com
Compressed Air Testing Services TRI Air Testing Inc. Compressed Air Testing Sop The purpose of this sop is to outline the procedure for testing the. this standard operating procedure outlines the process for monitoring compressed air and gases used in pharmaceutical production facilities. with the compressed air testing sop template, you can: — standard operating procedure to check the viable count of compressed air and nitrogen gas used in. Compressed Air Testing Sop.
From www.a1-cbiss.com
AIRQUAL1 Compressed Breathing Air Testing Kit BS EN12021 Compressed Air Testing Sop this standard operating procedure outlines the process for monitoring compressed air and gases used in pharmaceutical production facilities. — standard operating procedure to check the viable count of compressed air and nitrogen gas used in formulation of. procedure for compressed air quality testing 1) purpose. this standard operating procedure outlines the testing of compressed air and. Compressed Air Testing Sop.
From www.vrogue.co
Work Safety Tips When Using Air Compressors Infograph vrogue.co Compressed Air Testing Sop — applicable for monitoring of compressed air and gases (nitrogen, oxygen and carbon dioxide) in different production. procedure for compressed air quality testing 1) purpose. The purpose of this sop is to outline the procedure for testing the. this standard operating procedure outlines the process for monitoring compressed air and gases used in pharmaceutical production facilities. . Compressed Air Testing Sop.
From www.industrialair.co.nz
Compressed air quality standards Compressed Air Testing Sop tri has developed suggested direct and indirect product contact compressed air/gas guidelines based on practical common system capabilities such as. this standard operating procedure outlines the process for monitoring compressed air and gases used in pharmaceutical production facilities. Streamline the testing process and ensure consistent results. — applicable for monitoring of compressed air and gases (nitrogen, oxygen. Compressed Air Testing Sop.
From www.airbestpractices.com
Compressed Air System Commissioning Part 3 Testing Compressed Air Compressed Air Testing Sop Streamline the testing process and ensure consistent results. procedure for compressed air quality testing 1) purpose. this standard operating procedure outlines the process for monitoring compressed air and gases used in pharmaceutical production facilities. — standard operating procedure to check the viable count of compressed air and nitrogen gas used in formulation of. The purpose of this. Compressed Air Testing Sop.
From www.atheneq.com
Compressed Air Testing Device atheneq com Compressed Air Testing Sop with the compressed air testing sop template, you can: Streamline the testing process and ensure consistent results. tri has developed suggested direct and indirect product contact compressed air/gas guidelines based on practical common system capabilities such as. procedure for compressed air quality testing 1) purpose. — applicable for monitoring of compressed air and gases (nitrogen, oxygen. Compressed Air Testing Sop.
From www.scribd.com
03.SOP For Air Compressor PDF Chemical Engineering Energy Technology Compressed Air Testing Sop procedure for compressed air quality testing 1) purpose. this standard operating procedure outlines the testing of compressed air and nitrogen gas for viable counts in pharmaceutical formulations. The purpose of this sop is to outline the procedure for testing the. this standard operating procedure outlines the process for monitoring compressed air and gases used in pharmaceutical production. Compressed Air Testing Sop.